
(Illustration by Brad Yeo)
John B. Goodenough, SM’50, PhD’52, the father of the lithium-ion battery, sparked the wireless revolution. Now, at 94, he’s working on the next breakthrough.
John B. Goodenough can still remember, word for word, what one of his professors told him when he arrived on the University of Chicago campus 70 years ago: “I don’t understand you veterans,” said John A. Simpson, then a new instructor fresh off the Manhattan Project, later a pioneer in the study of cosmic rays. “Don’t you know that anyone who has ever done anything significant in physics had already done it by the time he was your age; and you want to begin?”
Goodenough, SM’50, PhD’52, breaks into his hooting lingering laughter that can be heard down the hall from his top-floor office in the engineering building at the University of Texas at Austin. He’d come to UChicago with a government-funded fellowship and “a good enough undergraduate record at Yale” (he rolls past the pun) that Simpson took him on. Besides, Goodenough had already decided that if he ever got the chance, he was going to study physics and was going to do something significant.
Today Goodenough is best known as the father of the lithium-ion battery. The rechargeable, lightweight battery is found in nearly all portable electronics, from power tools and medical devices to smartphones and laptops.
The battery made such mobile devices possible; it “revolutionized consumer electronics with technical applications for portable and stationary power,” according to the citation for Goodenough’s 2011 National Medal of Science.
Now 94 and the Virginia H. Cockrell Centennial Chair in Engineering at UT–Austin, Goodenough arrives in his lab every morning before 8 a.m. With a small flock of graduate students and postdocs, he’s working on a new battery to reduce our use of fossil fuels—and the greenhouse gases created as they’re converted to electricity—by providing a reliable, efficient way to store and transport wind and solar energy.
“We have to, in the near future, make a transition from our dependence on fossil fuels to a dependence on clean energy,” he says. “So that’s what I’m currently trying to do before I die”—to leave behind a cleaner, better world.

Born in 1922, Goodenough grew up outside New Haven, Connecticut. His parents’ “mismatched” marriage made for a difficult home life and Goodenough struggled with undiagnosed dyslexia (back then, he says, “you were just a ‘backward student’”). But he was determined to follow his older brother away to boarding school, so “I taught myself to write so I could write the [entrance] exam.” He was given a scholarship and at age 12 entered the Groton School in Massachusetts. The rigorous and highly structured education did him good, he says, and he was accepted to Yale in 1940.
Goodenough had almost completed his undergraduate degree in mathematics when he was called to active duty in 1943 as an Army meteorologist. (He had volunteered for the role at a professor’s suggestion; “I didn’t want to be a hero,” he says with a hoot.) The next spring he was granted a bachelor’s degree after Yale gave him credit for an Army meteorology course. He didn’t need to see combat to “realize the stupidity of war,” Goodenough says. Service left him with “the need to somehow do something for everyone”—to contribute, even in a small way, to the greater good. He thought his dyslexia would rule out a career in law or politics and had begun to take an interest in physics. The field was moving forward rapidly, and reading Alfred North Whitehead’s 1925 book Science and the Modern World convinced him “that much of the intellectual ferment of my generation would be in science,” he wrote in his autobiography in 2008. “I decided I should study physics if I ever had the opportunity.”
Shortly after the war that opportunity arrived in the form of a surprise telegram. Federal funds had become available to send a select group of returning Army officers to Chicago to do graduate work in the physical sciences. Unbeknownst to Goodenough, a Yale professor had submitted his name. “My debt to Professor Miles is profound,” he wrote in his autobiography.
Goodenough enrolled at UChicago. Going back to school after several years in the Army was a challenge, especially studying a mostly new subject—and especially under Enrico Fermi, whom Goodenough remembers as “old school.” His professors lectured only on topics that interested them, and he was expected to fill in the gaps independently. The professors were also not allowed to collaborate with students on their theses. During their first meeting, Goodenough’s adviser, solid-state physicist Clarence Zener, told him, “You’ve got two problems. The first is to find a problem and the second is to solve it. Good day.”
Goodenough did both, writing his thesis on how and why the structure of hexagonal metal alloys changes with the concentration of the conduction electrons, and got a PhD in 1952. “You see,” he says of Zener, “he did his job very well.”
After graduation Goodenough accepted a position at the Massachusetts Institute of Technology’s Lincoln Laboratory, established with federal funds in 1951 to build the country’s first air defense system (what would become the Semi-Automatic Ground Environment system, or SAGE). Goodenough’s team, tasked with improving memory capabilities in early computers, developed ceramic magnetic memory cores that enabled the first random-access memory (RAM); it was Goodenough’s first foray into chemistry and materials engineering. He worked at MIT Lincoln for more than two decades, investigating magnetism, cooperative orbital ordering, and d-electron behavior. His work describing electron exchanges between atoms and the resulting Goodenough-Kanamori rules laid the foundation for future design of magnetic materials and aided the development of computers.
Batteries have been around since 1800 when Italian scientist Alessandro Volta used copper, zinc, and salt water to create a device that could reliably turn chemical energy into electrical energy. Batteries store and release energy by means of chemical reactions that alter the chemical state of their two electrodes, the anode and the cathode. Ions are sent from anode to cathode through an electrolyte inside the battery, while electrons are forced to travel through a wire outside it, where they do work like lighting a bulb or powering a car. In rechargeable batteries, the original chemical state of anode and cathode can be restored by applying an external voltage between the electrodes, reversing the chemical reactions and allowing the battery to be reused.
The first rechargeable battery was the lead-acid battery. Invented in 1859 and by the turn of the century used in telegraph machines, streetcar systems, and electrical lighting substations, lead-acid batteries are relatively heavy and low voltage but cheap and dependable. Many of the earliest cars ran solely on these batteries, but a lead-acid battery can’t compete with the internal-combustion engine for speed or driving range. After the invention of the electric self-starter that replaced the hazardous hand crank, gas-powered cars like Ford’s Model T became safer and more practical. Lead-acid batteries were relegated to just starting the gas engine, and all-electric cars all but disappeared from American roads.
But by the 1960s, smog had engulfed several US cities and oil shortages in the early 1970s sent gas prices shooting up—along with concerns about American dependence on imported fuel. Even before the oil crisis of 1973, American consumers seemed ready to try battery-powered vehicles again; by 1972 General Motors, Ford, Chrysler, and American Motors (and automakers in Germany and Japan) all had electric cars in the works.
Meanwhile the US government began to increase funding for research into renewable energy and energy efficiency technologies. It was becoming clear, Goodenough wrote in his autobiography, “that our dependence on foreign oil was making the country as vulnerable as the threat of ballistic missiles from Russia.”
Goodenough was one of the scientists invited to monitor a 1966 breakthrough at Ford: a sodium-sulfur car battery that inverted the lead-acid design. The molten sodium anode and molten sulfur cathode, coupled with a solid ceramic electrolyte, formed a battery that was both lighter than, and had 15 times the energy density of, a lead-acid battery; it could power a car for up to 82 miles on a single charge. The battery was ultimately deemed impractical for use in automobiles—the molten electrodes meant it operated at 300 degrees Celsius—but the experience introduced Goodenough to electrochemistry and ignited his interest in battery science.
Ford’s breakthrough inspired other scientists too, at a time when federal and corporate money was pouring into battery research. One of the first completely battery-focused research departments was at Chicago-Affiliated Argonne National Laboratory, and among its first tasks was trying to improve on the same type of high-temperature batteries as the one Ford had developed. Especially after the 1973 oil embargo, the problem was clear and there were “lots of people looking for the answer,” says Argonne senior scientist and University of Illinois at Chicago physics professor George Crabtree, who now directs Argonne’s next-generation battery research.
About the same time, however, the Air Force funds supporting Goodenough’s work at MIT Lincoln started to dry up; Congress had decided that research financed by the armed forces should have practical military applications, and energy research didn’t fall into that category. Goodenough began exploring his options, including an offer to start an energy institute in prerevolutionary Iran. He—and his wife, Irene Wiseman Goodenough, EX’49, whom he met and married while at UChicago—were relieved when he was also invited to head the inorganic chemistry laboratory at Oxford University. In 1976 he joined the Oxford faculty and turned his attention to electrochemistry, including batteries.
That same year, Exxon patented the world’s first lithium-based battery, designed by M. Stanley Whittingham. Lithium is the lightest metal on the periodic table, and its positive ions conduct well in the battery’s organic liquid electrolyte. The low weight and large voltage capacity of Whittingham’s battery, together with the fact that it was designed to work at room temperature, made it a major breakthrough. However, as the battery charged and discharged, the surface of the lithium metal anode became rough, eventually spawning long narrow fingers, or dendrites, of lithium. These grew across the electrolyte and, when they touched the cathode, caused internal short-circuits that could make the battery explode.
Goodenough’s previous work with metal oxides and ion conduction made him think he could improve on Whittingham’s design. Whittingham’s battery used a layered sulfide cathode that allowed the insertion and extraction of large amounts of lithium between its layers (a process scientists call intercalation). Goodenough reasoned a layered oxide cathode would react similarly, providing a higher voltage that would enable a significantly higher energy density.
In 1980 Goodenough completed his lithium-cobalt-oxide cathode. There was little interest in his innovation at first, he says, because of skepticism about building a discharged battery. Meanwhile, scientists in Japan and Switzerland were showing that lithium can be intercalated reversibly into graphitic carbon, which is also layered. This offered the anode needed to go with Goodenough’s oxide cathode. The resulting lithium-ion battery cell safely gave a voltage of 4 volts, compared with 2.4 volts from Whittingham’s cell. Moreover, the battery, when made to industry standards with internal safety features, runs a very low risk of overheating and exploding.
Engineers at Sony recognized the potential of the breakthrough. In 1991 they commercialized a battery using Goodenough’s cathode, which ended up sparking the mobile revolution. With that cathode, Steve Levine wrote in The Powerhouse (Viking, 2015), Goodenough “outdid all that Ford, Argonne, and Whittingham had accomplished.”
Goodenough’s original lithium-cobalt-oxide cathode structure is still used in the lithium-ion batteries found in almost all personal electronics like smartphones and tablets. He’s since made several improvements on the technology; batteries using a lithium-manganese-oxide cathode, developed in his lab and refined at Argonne, are now used in many electric cars. His lithium-iron-phosphate cathode, completed in the 1990s, is found in most modern power tools. When he was tinkering with different oxides back at Oxford, Goodenough had no idea of the impact his battery would have. “I just knew it was something I should do.”
The battery and Goodenough’s subsequent improvements on the technology have earned him a host of awards. In addition to the 2011 National Medal of Science, he received the Japan Prize in 2001 and the Enrico Fermi Award in 2009. Organizations from Thomson Reuters to the American Chemical Society have named Goodenough a probable contender for a Nobel Prize.
Goodenough gave away his patents on layered and spinel oxides and the value of the patent for his olivine oxide was limited by a costly lawsuit, but lost royalties don’t concern him. He has enough for what matters. When he was named a recipient of Israel’s $1 million Eric and Sheila Samson Prime Minister’s Prize for Innovation in Alternative Fuels for Transportation last October, he used his share to help fund his lab’s research. And he recently endowed a scholarship at UT–Austin in memory of his wife, Irene, who died in January.
Goodenough moved to Texas in 1986, shortly before Oxford’s retirement policy would have forced him out. Thirty years later, he is focused on the kind of pure research he was doing at MIT Lincoln, examining the electronic properties in transition metal oxides and experimenting with superconductivity. The incremental improvements he’s been making in the size and performance of the lithium-ion battery bring in funding, says Goodenough, but he’s more interested in exploratory, outside-the-box research that will lead to true innovation.
At his age, “you don’t have much time left, and you really want to be able to solve the problem,” says Goodenough. “And I think we’re on the cusp of being able to do it.”
Goodenough is an adviser of the Argonne-led Joint Center for Energy Storage Research (JCESR), the Department of Energy–funded collaboration dedicated to improving battery technology and finding new energy storage technologies. Matthew Terrell, deputy laboratory director for science at Argonne and the dean and Pritzker Director of UChicago’s Institute for Molecular Engineering—a partner in the JCESR project—recently sat in on a JCESR advisory board meeting with Goodenough. “He had the most insightful, and probably the most numerous, suggestions for JCESR,” says Tirrell. He “has a kind of insight and intuition into inorganic materials science that is really incomparable.”
That deep knowledge, coupled with a willingness to work outside traditional disciplines, is needed to make the next big step beyond lithium-ion technology, says Tirrell. IME, which works on real-world applications of molecular-level science, has chosen energy storage and harvesting as one of its five main areas of focus. Because most modern batteries contain inorganic electrodes, organic electrolytes, and in some cases polymer separators, battery development requires expertise from different fields. At UChicago “we have experts here now on electronic materials, electronic structures, and transport materials, as well as inorganic materials,” says Tirrell—all the elements needed to make a better battery.
The impact would be significant. Electric grids and transportation together account for about 70 percent of total US energy use. It’s possible that one day all this energy will come from clean electric sources channeled through batteries, but it will require a dramatic breakthrough in battery technology, says Crabtree, JCESR director and Argonne senior scientist. Practical, affordable electric cars and large-scale storage for electric grids would require fivefold improvements in battery performance and cost, demands the lithium-ion battery just won’t be able to meet. “So you have to look for other batteries.”
JCESR scientists are experimenting with multivalent ions to shuttle more electricity between cathodes, and are working toward the elusive lithium-sulfur battery to make the needed leap. “The very best lithium-ion battery you could imagine is only a fifth as good as the very best lithium-sulfur battery you could imagine,” says Crabtree, but significant chemical barriers remain. Technologies like lithium-sulfur batteries may be 10 to 15 years away, Crabtree says. Still, the goal is “definitely within sight.”
Like his colleagues in battery science, Goodenough is looking everywhere. “I have learned to be open to surprises,” he says, to “not have preconceived ideas or close your mind from listening to what might work.” For example, a glass electrolyte brought to him recently by University of Porto engineering physics professor Maria Helena Braga caught his attention, and together they have shown that her electrolyte can be plated or stripped reversibly with metallic lithium or sodium, paving the way for use in a battery. Goodenough is also exploring alternative cathodes for both lithium and sodium batteries. He’s optimistic that at least one of the many projects being developed around the globe will lead to an important advance in the next year. No one’s sure where the next breakthrough will come from, “but it’s not impossible,” Goodenough says. He has faith.
A large tapestry of the Last Supper hangs in Goodenough’s lab, and religious artifacts share space in his office with souvenirs he and his wife collected over years of world travel. Goodenough’s father was a professor of the history of religion and as a student at Groton, where the headmaster was a proponent of “muscular Christianity,” he was expected to attend religious services regularly. But he didn’t fully embrace his faith until one night at Groton, when a dream helped him “get the metaphor,” he says. “I understood: God is love.” “Of course that’s what [the Bible] says all the time,” he says. “But it was the first time I experienced the love and I almost jumped out of my bed, I was so excited.”
The feeling has stayed with Goodenough for more than eight decades. It’s at the foundation of his work with batteries and his current quest for a superbattery. This is how he’s loving his neighbor, and his God, he says—by using his talent and working with his colleagues to create something that could help safeguard the planet and improve people’s lives.
But really, he adds, that’s what scientists do. “They want to understand nature so they can serve it. And they want to understand nature so they can, in conformity with nature, do something for their fellow man.”
Schematic of a lithium-ion battery
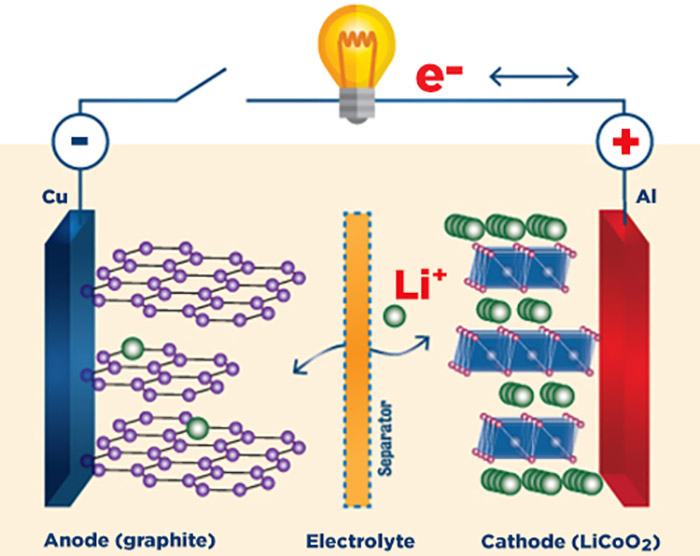
The first commercial lithium-ion battery, featuring Goodenough’s lithium-cobalt-oxide (LiCoO2) cathode, hit the market in 1991. When the battery is charging, positive lithium ions, represented by green circles in the schematic, move from the cathode to the anode. When the battery is discharging (producing the energy that does work like powering a smartphone), the positive lithium ions move back through the electrolyte while electrons (e-) are forced to travel around the circuit, generating an electric current. Lithium-ion batteries with this same basic design continue to be used in most personal electronic devices.
Updated 07.21.2023 to provide the University affiliation for Goodenough’s wife, Irene Wiseman Goodenough, EX’49.
