Photography by Robert Kozloff
Scientists have been at sea about how to extract uranium from the oceans, but a PhD student’s method shows promise.
The oceans are full of uranium. Altogether, they contain an estimated four billion metric tons of the silvery white metal—nearly 1,000 times more than all known terrestrial ores, and enough to fuel the nuclear power industry for centuries. And that’s important, given the growing need for energy sources to replace greenhouse-gas-emitting fossil fuels.
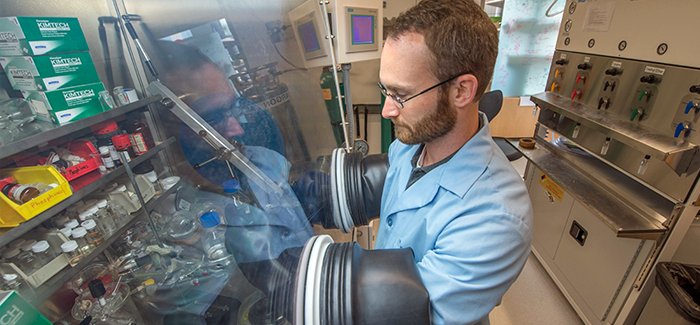
The uranium in the oceans could allow more use of nuclear power. The difficulty has always been finding a feasible way of extracting it from seawater. Carter Abney (above), a PhD student in chemistry, may have found one. Last fall he won a first place prize in the US Department of Energy’s 2014 Innovations in Fuel Cycle Research Awards competition for his contributions to a new method to harness uranium from seawater, using crystalline materials called metal-organic frameworks, or MOFs. These are compounds of metal ions and organic molecules; they function like proteins, with binding sites to attract uranium. MOFs have been designed for drug delivery within the body, gas separation and storage, and as catalysts for chemical reactions. Abney’s work, with UChicago chemist Wenbin Lin, was the first to use MOFs to extract uranium from seawater. In his study, they absorbed slightly more than 20 percent of their mass in uranium.
The same method can also help make nuclear power cleaner, by expediting its decay to safe levels. Materials made from MOF precursors can separate out highly radioactive elements called minor actinides. “You essentially consolidate the radioactivity into a condensed and stable form, which is more amenable to long-term storage and disposal than liquid waste,” Abney says.
