[Left] Seeman invented the field of DNA nanotechnology. (Photo by Jeff Singer)
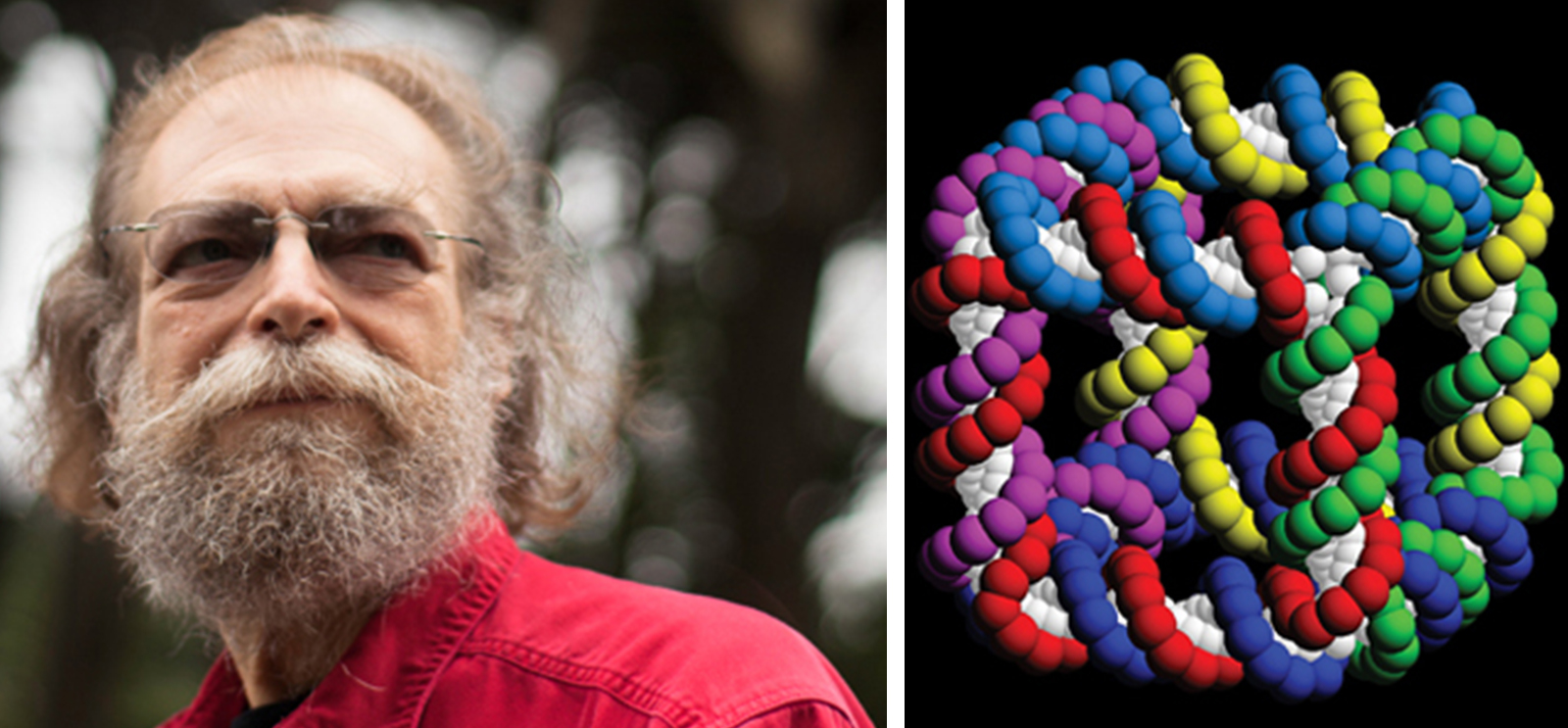
[Right] Seeman’s cube, based on research he published in Nature in 1991, “put DNA nanotech on the map.” (Illustration courtesy Ned Seeman)
Nadrian Seeman, SB’66, uses DNA not to study biology but as a building block for nano-tiny structures.
Nadrian Seeman walks to the podium to tell an audience of assorted scientists in Washington, DC, how to build things out of DNA. His first slide is his title, "DNA: Not Merely the Secret of Life"; his second slide is a naked lady. She's The Dream by Henri Rousseau, and she's sitting, pale among ferns and lotuses, benignly observed by lions, birds, and snakes. She represents biology, Seeman says, which "is not what we're talking about." For Seeman DNA is not just the genetic material by which life reproduces itself but a programmable Tinkertoy he arranges into structures that are nano-tiny—Tinkertoys the size of nanometers, a billionth of a meter. He calls this work "structural DNA nanotechnology."
Seeman, SB'66, looks out at the audience, the annual conference of the American Association for the Advancement of Science, straight on—Midwestern direct, a little amused, and not altogether sweet—and briefly reminds his listeners how DNA reproduces: its twin strands separate, then each becomes a template for its complementary strand. "Anybody who's been to kindergarten since 1960 knows this," he says. He explains how the original and complementary strands snap together: "get these guys naked in a pot, and they pair up." He shows how he's used DNA's complementarity to form the structures he wants it to form: squares, triangles, cubes, rhombohedrons. He's taken 30 years to make these structures, but now, he says, "I know where every atom is on these guys."
He doesn't say that he holds the Margaret and Herman Sokol chair in chemistry at New York University, nor that in 2010 he was a cowinner of the Kavli Prize in Nanoscience—the biannual prize, designed to be the field's Nobel, was first awarded in 2008. Nor does he say that he created the field of structural DNA nanotechnology in the first place.
He does say that structural DNA nanotechnology now has goals as diverse as nanoelectronics for faster computers and for nanorobots that pick up nanoparticles and walk like inchworms or do somersaults. Seeman runs through his slides, most of which show dense science, some of which show art that illustrates the science, and some of which show science so beautiful it looks like art. He began his research, he explains, out of frustration. He'd always been interested in what he calls "the edge of life," meaning the large biological molecules that carry information, the molecules of life. To study those molecules, including DNA, he needed to make crystals of them, but three years into his first faculty job, he "had managed to crystallize nothing," he says. So "to save my butt," he decided that, rather than trying to crystallize DNA himself, he would make DNA make the crystals for him.Seeman's hair is wild, his shirt is blue, and his jacket is sort of orange. He's overly direct and a little profane. All in all, he looks and talks as though he lives on the happy edge of anarchy.
Seeman was born in 1945; his mother was a schoolteacher and his father a salesman. His mother wanted to name him for her father, Nathan, but her father hadn't liked his name, and besides, she wanted an unusual name that would build character. So his mother made one up: Nadrian. Nadrian didn't like his name either and called himself Ned. He grew up in the Chicago suburb of Highland Park, an only child, a quiet boy who liked reading. In eighth grade Seeman was put into high-school algebra classes—Sputnik had gone up, the country panicked about its scientific competitiveness, and the government sped up educating scientifically adept children—and he continued to be fast-tracked through advanced math and science. He was good at both, but the class that got under his skin was biology.
His teacher, John E. Broming, began the study of life at the bottom: how atoms bonded to form molecules, how molecules were the components of cells, and how cells formed tissues, then organs, then organisms. For Seeman, the information was galvanizing: biology was fundamentally chemical, chemistry was fundamentally physical, and physics was just plain fundamental, and you could choose the level at which you wanted to work, and all of it, Seeman thought, went back to atoms. Toward the end of the course, Broming told his students about a biological molecule, whose structure was discovered in 1953 by James D. Watson, PhB'46, SB'47, and Francis H. C. Crick, called deoxyribonucleic acid, DNA, that somehow or other was the center of life. The information was vague, but Seeman was impressed by the vagueness too: the center of life was a molecule?
He graduated from high school in three years, at age 16. His parents wanted him to stay local for college, so he applied to the University of Chicago. For the first time he met other students from all over the country, all as smart as he was or smarter, all holding forth on interesting subjects he knew nothing about. "I was getting dipped in the mental peppermint," he says, "that every first-year student at Chicago gets dipped in."
His third year, he took a biology course and learned more about DNA. Each strand in the double helix is a lineup of four different chemicals—four combinations of hydrogen, carbon, nitrogen, and oxygen—called bases. The base adenine recognizes the base thymine; guanine recognizes cytosine. The complementary bases click together and spiral up into a new double helix. Atoms get together into bases, bases into DNA molecules: Seeman liked the logic.
That same year, Seeman took biochemistry—his major, although he didn't much like it. The field seemed focused less on chemistry as a foundation of biological information than on the biochemical cycles of nutrition and metabolism, one small molecule being turned into the next small molecule, forming pathways, cycle after cycle drawn on huge charts. The charts required a lot of memorization, and Seeman could imagine nothing more boring. He did well enough to graduate but no better.
He liked the idea of being a researcher, so with bad grades but good GREs, he applied to graduate schools in chemistry. He got into none of them. So he arranged to stay on at Chicago and take biochemistry courses again. It didn't go much better. The graduate adviser, John H. Law, said he should consider a new program at the University of Pittsburgh in biological crystallography. This time he got in.
Crystals are solids whose atoms form an orderly pattern; they're geometry in action. Consider salt: alternating atoms of chlorine and sodium, stacked so the pattern continues, sodium always next to chlorine in every direction and in three dimensions. Crystallography is finding that pattern, that structure, and where in the structure each atom sits. When Watson and Crick figured out DNA's helical structure, they did it largely with crystallography. Seeman had barely heard of crystallography, but it appealed to his sense of order and symmetry, it was fun, and he turned out to be good at it. He earned his PhD in three years.
In 1972 Seeman went to MIT for his postdoc. He'd been working on the crystal structures of RNA and verified a proposal by Watson and Crick about the way specific bases paired up, in detail at the atomic level. That work won him the 1974 Sidhu award from the Pittsburgh Diffraction Society. The award came to a net $87 ($100, less a meeting registration fee and a banquet ticket to pick up the award), but it was his first professional recognition. Later Nature called his research the "missing link" of nucleic acid structure.
Two years after winning the Sidhu, Seeman was searching for a faculty position. The job market for all crystallographers, not just the biological ones, was bad: of the hundred crystallography postdocs looking for jobs that year, only six would succeed. Seeman found himself kicking his bedroom wall: he'd been working for almost a decade, he'd gotten some recognition, yet he couldn't land a job. Finally, in 1977, by the time the hole in the bedroom wall was a half meter wide, he was offered a position at the State University of New York at Albany in the biology department.
The job was a bad match. His fellow biologists didn't share his appreciation for the chemistry and physics of biology; he felt intellectually isolated. He had longish hair and wore sneakers and jeans at a time when senior male faculty were more likely to wear jackets and ties. He was a bad match for the biology graduate students too; they were more interested in the hot new field of cloning, and they weren't much attracted to Seeman's crystallography. Without graduate students, he couldn't run a crystallography lab. Meanwhile, the field of biological crystallography had developed, and now crystallographers spent less time characterizing the structures of crystals and more time growing them. So although Seeman's strength was quantitative and analytical, and although he hadn't adequate manpower, he needed to spend time growing his own crystals.
He couldn't do it. Growing salt crystals is easy; growing biological crystals is high art. The DNA molecules he wanted to study rarely form crystals in nature. The lucky researcher could force their crystallization by controlling precisely every single thing about the process—temperature, acidity, concentration. If not, the molecules took whatever shapes they wanted to, and time after time they didn't want to be crystals. He'd have lunch every day with his friend Ken Karlin, and in addition to complaining about the administration, worrying about meeting women, teaching the more conservative Karlin how to swear, hoping they'd be recognized for good work, and knowing that good work took years, they fretted about whether they'd get tenure.
In 1980, two years away from the tenure decision, Seeman gave up on growing DNA crystals and decided instead to make computer models of them. In particular, he began modeling one specific step in DNA's metabolism. Two double helices unwind but only partially, leaving each helix with two free ends that join up with free ends of the other helix—a sort of a square dance where two couples break up and go off with each other's partners. The result is DNA restructured, no longer a double helix but a cross, a two-dimensional junction with four arms, four strands: branched DNA.
Seeman thought that branched DNA might have more than four arms. He went to the campus pub to have a beer and think about a six-arm junction, and for some reason he remembered a woodcut by M. C. Escher called Depth. In the image, ranks upon ranks of smiling fishes swim obliquely toward the viewer, their bodies parallel, each one with two lateral fins and a dorsal fin and a ventral fin that, along with the heads and tails, made each fish a six-arm junction. And if he joined the fishes—head to tail, lateral fins to lateral fins and vertical fins to vertical fins —they would make a three-dimensional scaffolding of little cages, an apartment building of tiny rooms. It would look like a crystal.
To construct the building, he'd have to join the six-arm junctions of DNA. He knew that genetic engineers could make DNA with what they called "sticky ends," building DNA helices with one strand longer, hanging off the end, and with a particular sequence of bases that in turn would join happily with its complementary sticky end. Put sticky ends on the branched DNA, Seeman thought, and they would "come together to form damn near anything, including crystals. So that was my little epiphany there."
That is, he could build an apartment building out of DNA, and into each room he could place any biological molecule so that whether or not the molecules wanted to, they'd be arranged into a crystal. By analyzing that crystal, he could be a crystallographer again. To celebrate, he had another beer.
By 1982 he had put together his tenure package—including his epiphany, mostly theory, with no successful experiments—and went off to Leiden University in the Netherlands to work with a chemist who offered to teach him to make his own sticky-ended branched DNA—which he did, he says, but "really badly." In the meantime, he'd been awarded tenure at Albany; a few years later, with $42,000 from the NIH, he bought a recently invented machine that made DNA to order. He treated the machine like a baby. In 1988 a collaborator of Seeman's became the chair of New York University's chemistry department and offered him a job in a more appropriate department with a lot of graduate students. Seeman took the job, moved his DNA machine to NYU, and he and his new students got to work.Building structures with DNA is a little like cooking with ingredients that have a mind of their own. First his team decides what they want to make and sketch out a design. Next they either build a physical model or run the design through a computer model to see whether DNA will let them get away with it: a biological molecule arranges itself into coils, folds, and contortions according to its own rules, which biologists know only imperfectly. Then they work out the sequence of bases they want. Strands with exactly that sequence are made either with Seeman's machine, or, often these days, ordered commercially. The strands, looped and twisted, are put in a solution and cooked to 70, 80, or 90 degrees Celsius until they straighten out and pair up. Over hours or days or weeks, they cool and condense out. If the DNA hasn't formed the intended design, Seeman and his colleagues have screwed up and need to go troubleshooting. "Nothing works the first time you do it," Seeman says. He cites Hofstadter's Law: "it always takes longer than you expect, even if you take into account Hofstadter's Law."
By 1991 they published in the journal Nature their first structure, a cube. But the cube couldn't be put with other cubes into a three-dimensional scaffolding for a crystal because it was, Seeman says, "a floppy piece of crap, and you can't make a crystal out of something that's floppy." Their next structure, a truncated octahedron, had some loose sticky ends that could in principle connect to the next truncated octahedron, but the first one took two years to make and it was floppy too. So they moved on to other shapes, trying to find ones that were interesting and nonfloppy and repeatable in a timely manner.
All along, Seeman and his team worked pretty much alone, until they published their floppy cube and it was admired in the popular-science press. Afterward, it became increasingly clear that, by treating DNA less like a biological molecule and more like an architectural widget, Seeman had created a new field, structural DNA nanotechnology.
The DNA structures qualified as nanotechnology because they were between a few and a hundred nanometers across. Nanotechnology itself is not really a distinct field, any more than "kilotechnology," the technology of kilometer-scale things, would be. It's an umbrella term for any number of tiny technologies made from any number of materials. Since 1998 its federal funding has been coordinated by the multiagency National Nanotechnology Initiative, and the science is housed in centers whose titles include words like nanomaterials, nanomanufacturing, nanobiotechnology, nanomedicine, nanoconductors, nanomechanical. In the mid-1980s nanotechnology had been more promise than practice, and when Seeman invented his subfield of DNA nanotechnology, he had only just heard the term. It made him feel like M. Jourdain in Molière's Le Bourgeois Gentilhomme, who was delighted to discover that he had been speaking prose all his life. By now Seeman was getting grants, he had good students, and around 1999, "after 25 years of one-night stands," he says, quoting an old show-business saying, "suddenly I'm an overnight success."
During the next decade the field blossomed. A scientist at another lab used some of Seeman's work and invented DNA origami, double helices folded to form a large flat surface, a floor, from which little snippets of sticky-ended DNA stick up. Seeman attached those snippets to strands of DNA that had been engineered into a little pivotable arm. Another scientist used a DNA origami floor and built a four-legged DNA spider to walk across it: the legs are sequenced to attach to the sticky ends of the snippets; then an enzyme cuts one leg's attachment, and that leg moves on to the next snippet; then the enzyme cuts the second leg's attachments, and it moves to the snippet after that. Seeman built such a walker with four legs and three arms, so it could not only crawl but could also pick up a nanoparticle and carry it to another snippet and either drop it off or pick up another one—a nanoscale assembly line.
Just what all this is good for is still a little unclear; a June 3 Science article says the field is still doing the basic science necessary for real-world applications. So far, scientists have learned to begin with bases that they string together into DNA strands that assemble themselves into three-dimensional structures that further assemble themselves into designed crystals—a level of control that seemed impossible 30 years ago when Seeman started. Today the basic science of structural DNA nanotechnology is practiced in more than 60 labs, all going in different directions. "The whole notion of this DNA Tinkertoy could be used for a gazillion things," says Seeman: DNA tweezers can open and close; DNA baskets can hold things. Labs make DNA nanotubes and nanoboxes; one lab makes DNA nanoflasks.
But no one has done what Seeman wanted to do in the first place: build a DNA apartment building with the same biological macromolecules in each room and do crystallography on that macromolecule. So far Seeman's lab has individual rooms shaped like triangles stacked in a building shaped like a rhombohedron. Seeman knows exactly what each room looks like and where every atom in it is because he designed it that way. Crystallography on DNA apartment buildings could be used to design drugs to fit certain targets, and some day such a building could assemble itself. He hasn't yet put the macromolecules into the rooms. "We're working on it," says Seeman. "Nothing is as easy as it looks."
With structural DNA nanotechnology now such a large field, Seeman takes comfort in the diffusion of responsibility: "We don't have to make all the discoveries," he says. "We don't have to make all the mistakes." Another comfort is personal: "I'm still the old man in the field, and I get a little recognition for that." In 1995 Seeman won the Feynman Prize, and in 2010 he shared the Kavli Prize, which was 5,000 times larger than his first $100 award. When he summarizes his career, he seems more impressed by his frustrations and near failures than by his eventual success. Still, prizes and a proliferating field—doesn't that feel good? "It does feel good," he says. "It's exciting that there's a whole field based on what I was thinking about, drinking a beer, in 1980."
