
(Illustration by Gwen Keraval)
Scientists are discovering how microbes not only make us sick but also keep our bodies working.
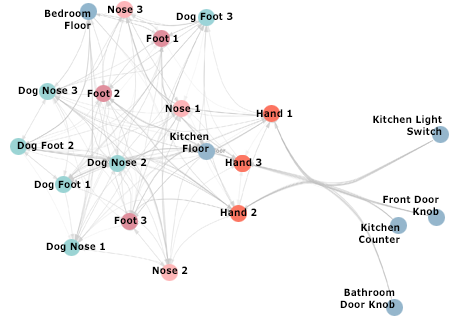
There’s a story that Jack Gilbert, a microbial ecologist at UChicago and Argonne National Laboratory, likes to tell about a bacterium called Enterococcus faecalis. It’s sort of a love story gone wrong. Squat and vaguely jellybean-like, measuring about three microns long, E. faecalis lives in the human gastrointestinal tract. Under normal circumstances, the relationship is friendly. It’s close. It’s what microbiologists call commensal, a term whose Latin etymology conjures up togetherness and a shared dinner table. “In its original state, just living inside your gut, this bug is totally harmless,” Gilbert says. “In fact, it’s beneficial. It helps train your immune system.” Your body wants it there, needs it there, has evolved to live with it. “It’s a natural part of your gut’s flora, your ecosystem.”
All that can change, though, when a person goes in for gastrointestinal surgery. Like, for instance, to remove part of the colon and stitch the remaining pieces back together, a routine treatment for colon cancer. Afterward, some patients develop what’s called an anastomotic leak. The seam where the bowel has been rejoined breaks open, and fluids from the intestine begin seeping into the body. It’s a rare complication, but it can be disastrous, sometimes fatal. Even after years of increasingly better materials—glues, staples, stronger stitches—and increasingly precise surgical techniques, anastomotic leak persists. Some surgeons opt to avoid the risk altogether by performing a colostomy that, unpleasantly, diverts fecal matter into an external bag.
The culprit, it turns out, is usually not the stitches or the surgeon; instead, it’s a particular strain of the otherwise commensal E. faecalis. In a study published this past May, Gilbert and John Alverdy, the Sara and Harold Lincoln Thompson Professor of Surgery, found that the bacterium creates small holes in the intestine at the surgical site, degrading the tissue and weakening the connection. In rats with anastomotic leaks, the abundance of E. faecalis ballooned 500-fold. “It becomes like a swarm of locusts,” Gilbert says of the microbe. “And it swarms directly to the site of damage in the cell wall, grabs hold of it, and starts to break down the collagen that the body is trying to use to repair the cell damage. It’s like going to the scaffolding on a new building and just ripping it apart. And the building falls down.”

But why? What makes this friendly bug turn against its host? The answer, Gilbert says, underlines an increasingly inescapable need to reimagine the way medicine is practiced. Not just surgery, but all medicine. And—now that he’s talking about it—not just medicine, but modern life more broadly. The cities we build, the buildings we work in, the food we eat, the homes we keep, the environments where we live our lives and raise our children. All these factors affect the microbes living inside us, which in turn, scientists are discovering, can influence everything from obesity to Alzheimer’s to asthma.
So here’s what that colorectal surgery looks like to the microbe. When the patient comes to the hospital, the first thing doctors do is blast the intestine with radiation, to kill as much bacteria as possible. Then they pump in intravenous antibiotics to try to get rid of whatever remains. “Again, it doesn’t kill everything, but, again, it creates an incredibly hostile ecosystem,” Gilbert says. Then the surgery. Doctors cut open the colon, flooding the normally anaerobic gut with oxygen. “The oxygen makes the surviving anaerobic organisms panic,” Gilbert says. “It’s like poison gas.” Finally, doctors seal up the incision and the wound begins to heal itself, siphoning nutrients, namely phosphorus, from the intestine. Suddenly there’s not enough food for the microbes.
Gilbert is excited now, waving his arms a bit, shaking the air in front of him. He’s at a Starbucks down the street from his UChicago office, gulping down black tea before his next lecture. “Let’s look at it from the bacterium’s perspective,” he says. “It’s the last of its kind, right?” Having survived radiation, antibiotics, and oxygen exposure—and along the way having likely selected for a mutation that transforms it into a hardier strain of bug—E. faecalis now finds itself robbed of phosphorus and starving to death. “It’s like, ‘Oh my god, this is a horrible environment!’ So this bacterium, he’s normally your friend, right, but what do you think it does?” Gilbert asks. “It turns on mechanisms and pathways inside itself for nutrient acquisition.” And the best place to find those nutrients? “This lovely, nicely available healing-up area where the body’s sucking up all the phosphorus.” So that’s where the bug goes, and it attacks the wound. “And we call that an infection; we call that a pathogen. And we try to kill it off, without realizing what we did to make that pathogen appear.”
Humans are vastly outnumbered in their own bodies. For every human cell, there are 10 cells of bacteria. But until they’re born, babies are sterile. They leave the womb and pass through the birth canal, where they’re colonized by their mothers’ microbiota. After that, children pick up bacteria everywhere they go: from their parents and siblings and other people, from pets, food, clothes, floors, furniture, toys, plants, trees, dirt, and the air all around them. By the time children learn to walk, they’re enveloped, inside and out, by a massive, invisible kaleidoscope of microorganisms, 100 trillion or so. Those microbes—mostly bacteria but also some viruses and fungi—live in our mouths and blanket our skin; they congregate in our nasal passages and ear canals and on the surface of our eyes. More than anywhere else, they inhabit our digestive systems.
Taken together, these organisms are called the microbiome, and they are so pivotal to our health, both its function and dysfunction, that scientists have begun thinking of them as another organ. Indeed, about three pounds of every person’s biomass is microbial; that’s roughly the same weight as the human brain. Friendly microbes living happily in our bodies help train our immune system, help digest our food and absorb nutrients from it, and help keep pathogens at bay. But the role that E. faecalis plays in anastomotic leaks is only one example of what can happen when this complex and dynamic community of organisms falls out of balance.
UChicago scientists, including Gilbert, are researching the ways in which “dysbiosis,” a microbial imbalance inside the body, can lead to food allergies and inflammatory bowel disorders. Pathologist Alexander Chervonsky studies the link between an absence of certain microbes in the gut and the onset of type 1 diabetes and other autoimmune disorders. He’s also examining how the differing composition of male and female microbiomes may at least partly explain why autoimmune disorders strike women more often than men. Pediatrician Stacy Kahn has looked at how fecal transplants, which transfer gut microbes from one person to another, can be used to treat recurrent Clostridium difficile infections in children.
Geneticist Carole Ober is working to unravel the microbial influence on asthma. For decades, Ober has studied the Hutterites of South Dakota and the Amish of northern Indiana, two groups with nearly identical genetic ancestry—both are Anabaptists who live on communal farms—but strikingly divergent childhood asthma rates. At 15 percent, the Hutterites’ rate exceeds the national average, while the Amish Ober studies have almost no asthma at all. Her recent research points to the seemingly protective effects of dust, and the microbes within it, found in Amish homes. New research, not yet published, on which Gilbert is a collaborator, also points, he says, to differing traditional practices that have Amish children working out in the barns at a much earlier age than the Hutterite children.
These days news of the microbiome is everywhere, in scientific journals and newspaper accounts and scholarly symposia. Gilbert gives public talks on microbiome research constantly, sometimes two or three in a week, almost always to packed houses. At community colleges and in university classrooms, in auditoriums and at conferences and recently at a bar on Chicago’s North Side, where he balanced a beer in one hand and a microphone in the other while the Stanley Cup play-offs flickered across muted screens overhead and bartenders paused to listen in. People crowd in to his talks and stay late to ask question after question. How hard is it to change your microbiome with diet? (Pretty hard, but not impossible.) Are microbes involved in cancer? (Possibly—scientists are looking into it.) When will this stuff become part of regular doctor visits? (We’re working on it.) Do probiotics really work? (Sometimes; it’s complicated.)
Microbiome research is still in its early stages. A decade or more ago, genetics seemed like the key to understanding our biological fates. Find the gene and you’ll find the disease. But the picture turns out to be much more complicated. Genes are important, but not by themselves determinative. And the same DNA-sequencing technology that made possible the map of the human genome also made it possible to sequence and analyze the microbes in the human body. A whole new universe sprang into view. “When you think about where medicine has been for 100 years,” Gilbert told a classroom of Northwestern University public health students this past spring, “we know the 40 organisms that make us sick.” Those are the ones whose names are so familiar: tuberculosis, cholera, plague, smallpox, whooping cough. But there are millions of other microbes that scientists are only starting to identify, whose relationships to their human hosts we have yet to decipher.
In his talks, Gilbert blazes through a litany of recent studies demonstrating microbes’ powerful influence. How scientists took a particular organism from the gut of one fruit fly—animals all have their own microbiomes too, as do plants and soil and oceans and every other kind of environment, even man-made ones—and transferred that microbe to the gut of another fruit fly, thereby altering the type of mate the second fly preferred. How scientists working in China isolated a bacterium, Enterobacter cloacae, in a 385-pound man, and when they put it into the body of a normal-sized mouse, the mouse gained more than twice as much weight as a mouse on the same high-fat diet without the bacterium. How a study in Italy, which Gilbert coauthored, found that the microbial composition of obese subjects’ saliva seems to supress wine (and food) aromas, leading them to consume more.
Gilbert tells his audiences that a mouse pup engineered to be sterile, with no microbiome at all, grows up strange, not only physiologically—with an enlarged intestine—but neurologically too. Place a germ-free mouse inside a little box on a raised platform, and it won’t stay there and hunker down the way mice typically do, hiding from the owls and eagles; instead, it runs to the edge of the platform and quite often jumps off. “It becomes a little crazy,” Gilbert says. But when scientists implanted a microbiome in that same mouse, it reverted to normal, timid behavior.
Sometimes Gilbert opens up about his eight-year-old son Dylan, diagnosed a few years ago with autism, and how Gilbert, almost that very day, called up colleagues across the country to ask for their help, and to offer his, in studying the connection between microbes and autism, which is now a major focus of his lab. He and his colleagues, some of whom were already studying that connection, formed the Autism Microbiome Consortium. In December 2013 they published a companion paper to a study by scientists at the California Institute of Technology identifying a microbe, Bacteroides fragilis, that when administered to a mouse with an autism-like syndrome, reversed its symptoms.
“We’re able to essentially cure autism in this mouse model by changing the bacterial flora in the gut lining,” Gilbert told an astonished audience during a 2014 talk on campus. On the strength of that finding, and his own study demonstrating that a dog can exponentially increase the microbial diversity of a home—“they bring the outside inside”—Gilbert adopted a collie–golden retriever mix, which his children named Captain Beau Diggely. “We were primarily interested in what the dog could do for my son’s microbiome and how that could influence his behavior,” Gilbert said. “And it’s had a significant impact—over time.” He paused, adding, “And scientifically significant, not just parent significant.”
It’s easy to get carried away with all this. The promise seems so limitless. At a panel discussion on the microbiome during Alumni Weekend, Gilbert cautioned people not to get ahead of the science. “We can’t go in thinking this is the answer to everything.” So little is known, and the complexity is almost unimaginably vast. Mouse models are a long way from human clinical trials.
Still. Alverdy, who collaborated with Gilbert on the E. faecalis study and has spent more than 20 years analyzing the behavior of intestinal bacteria, says this research is perhaps the most significant happening now. “I believe that understanding the microbes is how we’re going to save the earth,” he says. “Really. Truly. They’re that important.”
For patients who undergo gastrointestinal surgery, and other procedures like bone marrow and organ transplants, recovery depends significantly on how fully their microbiome “reblooms” afterward: how many of their normal gut bacteria, which regulate their immune systems and keep pathogens at bay (remember, Alverdy says, in the wilderness of the body, bacteria are spitting antibiotics at each other all the time), manage to recolonize after the trauma and disruption of surgery. “When you come into the hospital and you have your tooth done or your eye, they whack you with antibiotics, and your bugs go, ‘What?!’” Alverdy says. “And they get wiped out for a while.” Usually, a few remain, from which the colony can regrow. But the bigger the surgery and the longer the hospital stay, the greater the chances that damage to the microbiome can become permanent.
“The people whose normal microbiome erodes are the ones that do the worst,” he says. “When their own normal good guys start to deplete, if they don’t come back, you’re going to have a real high incidence of not only your cancer coming back but also dying.” One solution might be for patients to “bank” some of their stool, containing a full array of their normal microbes, to be reintroduced to the gastrointestinal tract afterward, the same way patients used to bank their own blood before surgery. Another idea Gilbert and Alverdy are working on is to find a way to deliver a phosphate compound to the surgical site, so that nutrient levels stay high enough to keep the microbes fed while the wound heals. “To stop the bacteria from going pathogenic,” Gilbert says. “You wouldn’t say that’s a healthy microbiome exactly, but you’d say that microbiome is significantly less likely to go rogue and become virulent.”
In Alverdy’s research of postsurgical patients—both those who develop infections or other complications and those who don’t—he looks at which bacteria appear in the body after an operation, but also, he says, “who’s missing.” He sees the same scenario emerge again and again. “We do liver transplants and we give people immunosuppressant drugs and then they have temperatures after surgery and we give them more antibiotics and kill all the bad bugs,” he says. “And then more resistant bugs develop and we give antibiotics to kill all those resistant bugs.” The cycle continues: resistant bugs becoming more-resistant bugs. “And then the patient might reject their organ, and then we’ve got to give them more immunosuppression.”
He draws a breath. “I mean, think of what we’re doing to the tropical rainforest of our bodies.”
Antibiotics have saved millions upon millions of lives. During the 19th century, it was not unusual for a family to lose a child or two to infection. Life expectancy for an American born in 1930 was about 60 years. For a child born today it’s 79, and antibiotics have had no small part in that transformation. But scientists are also beginning to understand the toll those life-saving drugs take on the microbial organ. “What we like in infectious disease is, ‘Here it is! Here’s the bug! Kill it all the time and you’ll never have a problem,’” Alverdy says. “But we’re realizing that’s not how the microbiome works. The paradigm has to change. Because we’ve been killing things for a long time, and now we have a lot of new problems: multiple sclerosis, neurologic disease, Alzheimer’s, autism, inflammatory bowel disease, anastomotic leaks.”
UChicago gastroenterologist Eugene Chang, MD’76, the Martin Boyer Professor of Medicine, studies inflammatory bowel disease, a term that encompasses several conditions but primarily Crohn’s disease and ulcerative colitis. Fifteen years ago, Chang pivoted unexpectedly into microbial research and retooled his whole laboratory to pursue it. Studying a group of genes called heat-shock proteins, which protect the gut against injury like that caused by inflammatory bowel disease, he discovered that microbes provide the signals that regulate those genes. “They’re absolutely required for maintenance of this particular set of genes in our body. And without these genes, we’re susceptible to disease,” Chang says.
Recently Chang and postdoc Vanessa Leone found that gut microbes influence circadian rhythms, the sleep-wake cycle that plays a role in metabolism and tells the body when to burn energy and when to store it. “Vanessa found that over 1,000 genes in the liver”—which helps regulate the circadian clock—“are controlled by gut microbes. Think about how profound that is. This has got to be at least 10 percent of your genes in your liver.”
Those microbes, Chang and Leone found, are in turn influenced by what we eat. Modern Western diets—high in fat and carbohydrates, low in fiber and whole grains—alter the microbes involved in circadian rhythms, Chang says. “It ablates that rhythm, and that creates an imbalance that changes the metabolic set point of individuals,” the baseline rate at which the body burns calories. “We think it’s a mechanism that promotes obesity and metabolic syndrome.”
Meanwhile, working with researchers at Argonne and the University-affiliated Marine Biological Laboratory in Woods Hole, Massachusetts—the computational tools developed at MBL and Argonne’s high-through-put genome sequencing technology make possible much of the microbiome research at UChicago—Chang is learning that some patients aren’t “wired correctly” to react to pathogens in the gut. And those pathogens are associated with ulcerative colitis. “There’s almost certainly some kind of miswiring of their circuitry that leads them to respond inappropriately to microbes in general, but in particular maybe to these types of pathogens,” Chang says. The bad bugs seem to have genes that can generate a “cloaking device” that allows them to elude the immune system. This research is early and ongoing. “We need to get more patients,” Chang says, “but it’s very exciting.”
Some of the most promising discoveries have come in the realm of allergies. Particularly food allergies—peanuts, tree nuts, fish, shellfish, milk, eggs, wheat, and soy are the big ones—which have risen dramatically, and somewhat mysteriously, over the past two decades. Last year, UChicago immunologist Cathryn Nagler, the Bunning Food Allergy Professor, identified a particular class of gut bacteria, Clostridia, that seems to protect the body against allergies by preventing allergens from getting into the bloodstream.
Like her colleagues, Nagler blames, in part, the overuse of antibiotics, with its practice of wiping out the good bacteria with the bad. But for allergies, and perhaps other maladies as well, the trouble may begin much sooner, at the moment a person is born. Humans are evolved to get their starter microbial culture, their “sourdough bread,” as Gilbert calls it, from their mothers’ birth canals. But that only happens if they’re delivered vaginally. When children enter the world, they’re colonized by whatever microbial community they first touch. For those born by cesarean section, that initial contact is not with the mother’s birth canal but with her skin—or with the doctor’s. Both have very different sets of microbes. Clostridia typically aren’t among those bacteria that babies acquire in the birth canal—they arrive later, picked up from the outside world—but they rely on the founding microbes to establish a climate in the gut where they can grow. Says Nagler, “Cesarean section is a major environmental factor associated with allergic disease.”
A second blow to an infant’s microbiome may come in the form of sterile formula. “There’s this beautiful interplay between the microbiota and breast milk,” Nagler says. Gilbert, a collaborator of Nagler’s, often describes it in his talks. “Evolutionary transcendence,” he calls it. “A perfect probiotic.” The mother’s body “recruits” bacteria from the gastrointestinal tract, the vagina, the mouth and deposits them, intact and alive, in the breast duct. “So the breast milk comes out with a complex microbiome and a probiotic,” Gilbert says. “It’s not sterile. So not sterile. It’s designed to elicit an immune response in the baby’s gut, causing an improvement, a training of that baby’s immune system.”
But like antibiotics, C-sections have saved many lives. And some babies cannot be breast-fed. Nagler and her collaborators—biotechnologists, nanotechnologists, and pharmacologists among them—are working toward a probiotic that might protect children against food allergies. “The best chance of this working,” she says, “is to introduce it early in life.” During a child’s first two years, the microbiome is plastic; it’s still forming and changing and would be more receptive to the bacteria in a probiotic. “And that can shift its course.” In adults with a firmly established community of microbes, it’s harder for new bacteria to gain a foothold.
Probiotic is kind of a conjuring word these days. When Gilbert speaks in public, people almost always ask him about them. Does he recommend taking probiotics? Does he think they really work? Is that where all this research is headed? The answer is, essentially, yes and no. For specific disorders, like food allergies—or, perhaps, autism—probiotics carrying specific microbes might offer an effective solution, a good stopgap, he says, to strengthen or suppress the immune system as needed. But simply for maintaining robust everyday microbial health? There’ll probably never be any one pill or probiotic, or cup of yogurt, for that.
That’s because there’s really no such thing as a single, specific healthy human microbiome. Microbiomes vary widely, based on genetics, geography, diet, and lifestyle. Everyone has their own, and tending to it will require precision medicine, Gilbert says, therapies tailored to the individual. One of his far-off schemes involves a toilet that samples its user’s stool and monitors it for microbial changes. Every morning the toilet could calculate what kind of food would rebalance the bugs in a person’s gut that day. “I like that idea a bit more,” he says, “that we can treat the microbiome not by swapping out individual members”—in other words, giving probiotics to engineer a specific population of microbes for everyone—“but by treating the bacteria themselves as components that need to be kept happy.” He pauses. “So, a happy microbiome might actually be a real thing.”
Gilbert keeps talking. (“I’m a tangential human being,” he likes to say.) It turns out he does have ideas for other, broader improvements for our population-wide microbial well-being. Mostly those ideas revolve around a single imperative: more microbes, everywhere. Our lives are too sterile, he says, and counterintuitively, that leaves us more vulnerable. A study called the Hospital Microbiome Project demonstrates that idea. Starting in early 2013 Gilbert and a small army of grad students, postdocs, and research assistants spent a year taking a microbial census of the University’s new hospital pavilion, the Center for Care and Discovery. They arrived before the doors opened to patients, and with cotton swabs they took samples from floors, beds, linens, sinks, computers, nurses’ stations, and air vents, and, after the facility opened in February 2013, staff, patients, and doctors too. They repeated this several times every day for 365 days and watched as the hospital’s ecosystem of microorganisms changed over time. They’ll be analyzing the data until probably 2016, Gilbert says, but some early results are in.
“As soon as the hospital opened,” he said during one talk, “the human microbiome crept in, and it made it much more diverse than it was before, more microbial organisms, but a lot more human pathogens.” No surprise there, but Gilbert’s been thinking since then about how to reduce those pathogens—how to create a hospital ecosystem that’s less hazardous to the people inside it. More microbes, he thinks. “If I throw a pathogenic organism onto the floor and the floor’s perfectly sterile, it might die. But it will be viable for a period of time, and while it’s viable, it’s likely to be transmissible. But if I throw it onto the floor and the floor’s covered in a rich microbial ecosystem, it’s got nowhere to go. There’s not real estate for it to take up home.”
Humans evolved living in places covered with microbes. Only in the last century or so have we come so completely indoors. Today, most Americans spend 90 percent of their time inside; for babies that number is even higher. “We built these buildings to make them as biologically horrible as possible,” he says. “These environments are designed to kill bacteria and fungi. They’re dry. They’re full of nonporous surfaces. The temperature is controlled.” But it hasn’t fully worked: pathogens still get in, and if the environment is otherwise microbially dead, there’s plenty of real estate for them to cause havoc. “What if I could change that?” Gilbert said recently to a group of UChicago undergraduates. “What if I could create wood structures or carpets or buildings that were microbiologically active instead of being depauperate like they are now? What if we could change HVAC systems so we were all being constantly bombarded by a rich, diverse, healthy microbiome? That’s where we want to push the research.”
In December 2014 Gilbert and Canadian biologist Josh Neufeld published a short paper in PLOS Biology called “Life in a World without Microbes.” Humans have been trying to eradicate germs from their bodies and their environments ever since microscopes made it possible to detect them. What if they all just disappeared? At first, we might not notice, the authors write. But pretty quickly, things would start to go wrong. Gilbert and Neufeld describe rapidly accumulating organic waste, stagnating oceans and soils, a swift uptick in global warming. The biogeochemical cycles that circulate nitrogen, carbon, oxygen, and water throughout the planet would come to a halt. So would photosynthesis. Humans and animals would be born with shrunken hearts and lungs and neurological problems. Small pockets of people might survive for a while, but in the end we’d all be doomed.
Without our microbes, we’re just not ourselves.
