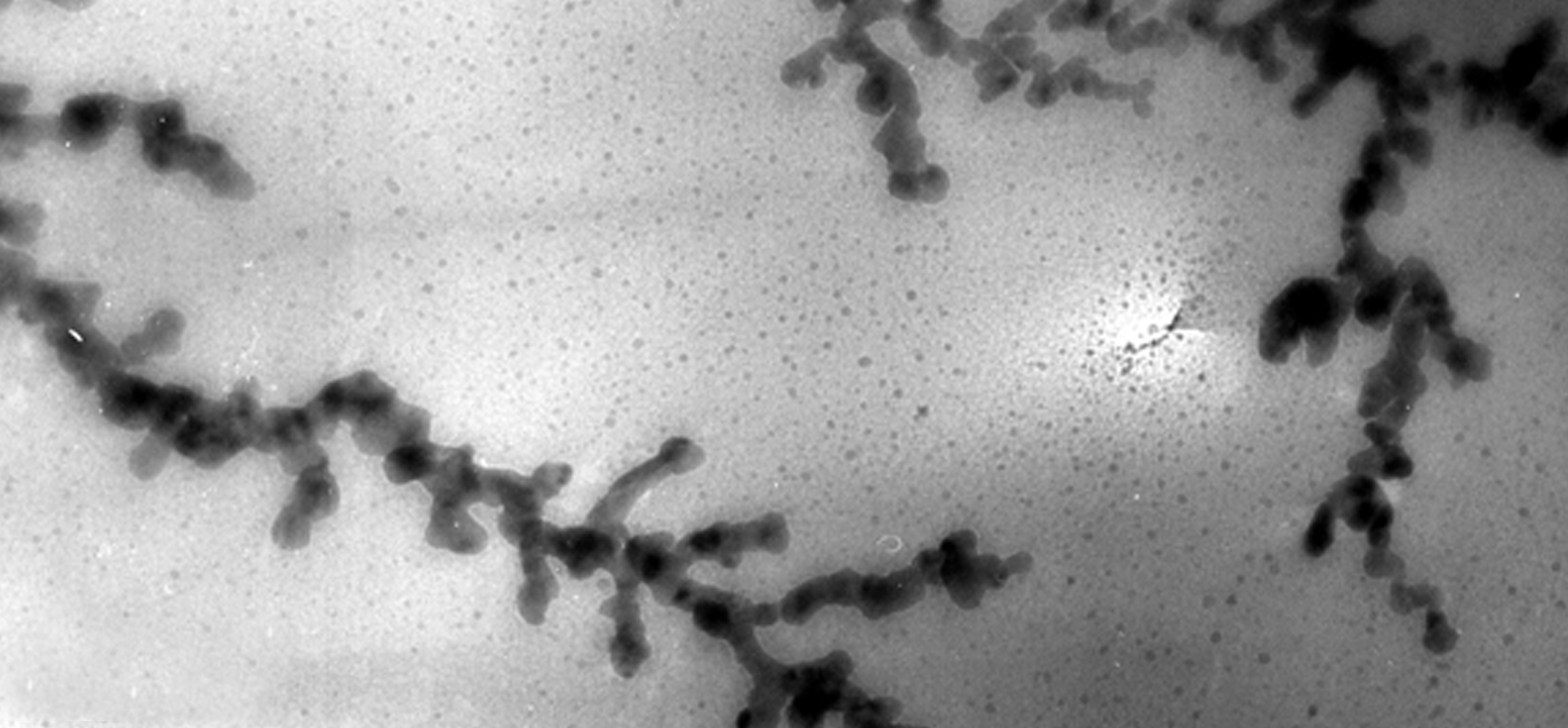
Lithium metal dendrite in a polymer electrolyte. (Image courtesy G. M. Stone/UC Berkeley and LBNL)
In 1966 the Ford Motor Company developed a sodium-sulfur battery, able to power a car for up to 82 miles. The battery was ultimately deemed impractical—it was prohibitively expensive and operated at 350 degrees Celsius—and Ford shelved the project.
But the battery sparked an interest in physicist John Goodenough, SM’50, PhD’52, then a researcher at MIT’s Lincoln Laboratory, who had been invited to monitor the project. “That invention came because someone found fast sodium-ion transport in a ceramic,” he says. Goodenough came to realize, “I know how to do it better.”
Today companies across the globe are still working on a better battery, many aiming to control what will be an estimated $300 billion global industry for electric vehicles. Goodenough, at 94, is also working on a better battery—one he hopes will help us not only switch to electric cars but also end our dependence on fossil fuels completely.
Goodenough, the Virginia H. Cockrell Centennial Chair in Engineering at the University of Texas at Austin, is widely known as the “father of the lithium-ion battery,” the rechargeable, lightweight battery found in nearly every portable electronic device, from smartphones and tablets to power drills.
As President Barack Obama put it when he presented Goodenough with a 2011 National Medal of Science, the lithium-ion battery “revolutionized consumer electronics with technical applications for portable and stationary power.” The battery—and Goodenough’s subsequent improvements on the technology—have won him a host of other awards, including the Japan Prize in 2001 and the Enrico Fermi Award in 2009. Organizations from Thomson Reuters to the American Chemical Society have named Goodenough as a contender for the Nobel Prize.
[[{"type":"media","view_mode":"media_original","fid":"3605","attributes":{"alt":"","class":"media-image","height":"375","typeof":"foaf:Image","width":"500"}}]]
Goodenough and President Obama. (Photo by Ryan K. Morris, courtesy the National Science and Technology Medals Foundation)
A battery consists of two electrodes—the anode and cathode—separated by an electrolyte medium. Batteries store and release energy through chemical reactions, which alter the state of the two electrodes. In a lithium-ion battery, the spontaneous reaction (discharge) leads to positive lithium ions and negative electrons migrating from anode to cathode. The quickest and easiest route is through the electrolyte within the battery, but a membrane separator allows only the lithium ions to pass. Electrons must travel outside the battery, creating a current that can power devices, before reentering the battery. Rechargeable batteries, like lithium ion, use external electricity to reverse the reaction and restore the electrodes to their original state.
[[{"type":"media","view_mode":"media_original","fid":"3604","attributes":{"alt":"","class":"media-image","height":"397","typeof":"foaf:Image","width":"500"}}]] (Adapted with permission from Goodenough J. B., Park K. S., 2013, JACS, 135, 1167−1176. Copyright 2016 American Chemical Society.)
In the late 1960s, Goodenough was working on a layered sulfide structure that reversibly intercalated (or accepted and stored) large amounts of lithium, which led to the suggestion that such sulfides could be used as the cathodes of a rechargeable lithium battery. Stan Whittingham, a battery pioneer at Exxon Mobile, explored this idea, demonstrating in 1976 the first rechargeable lithium battery of high energy density.
Yet Whittingham’s design had a major challenge to overcome: anode dendrites—microscopic fibers of lithium that emerged after several charge/discharge cycles— would grow across the flammable liquid electrolyte, causing an internal short circuit, leading to incendiary, even explosive, events.
Goodenough knew how to fix this problem: suppress lithium metal dendrites by using a more stable lithium metal oxide. By then head of Oxford University’s Inorganic Chemistry Laboratory, Goodenough drew on work he had done with layered oxides as part of the team that developed the first random-access memory (RAM) at MIT Lincoln. Assembled in a discharged state, his battery would use an anode that reacted reversibly with lithium from the cathode on initial charge.
In 1980 Goodenough completed a battery powered by his lithium-cobalt-oxide cathode. It could safely produce 4 volts of energy, compared to 2.4 volts in Whittingham’s battery. There was little interest in his innovation at first, says Goodenough, because of skepticism about building a battery in a discharged state. However, scientists in Japan and Switzerland had realized that lithium can be intercalated reversibly into graphitic carbon, and they demonstrated a safe, high-energy-density rechargeable battery with Goodenough’s oxide cathode and a graphitic-carbon anode. Engineers at Sony recognized its potential and in 1991 commercialized a battery using Goodenough’s cathode in its popular camcorders. Soon the lithium-ion battery became an indispensible part of modern life.
Since then Goodenough has continued to perfect the technology. “People make money on the lithium battery, so that means I can get funded to improve the batteries,” he says. But he also has his eyes set on a “super battery,” a new technology that will lead to mass-produced electric cars, large-scale storage for citywide electric grids, and a world that no longer runs on fossil fuels.
The breakthrough needs to happen soon, says Goodenough. Even before the 1973 oil crisis, it was “obvious,” he wrote in his 2008 autobiography, “that our dependence on foreign oil was making the country as vulnerable as the threat of ballistic missiles from Russia.”
Growing political instability and the looming threat from climate change has kept Goodenough coming into his top-floor office at UT Austin every day at 8 a.m., long after many of his peers have retired. “We have to, in the near future, make a transition from our dependence on fossil fuels to a dependence on clean energy,” he says. “So that’s what I’m currently trying to do before I die”—to develop the next generation of batteries in order to leave behind a cleaner, more stable world.
Recently Goodenough’s attention has returned to sodium batteries; sodium is more plentiful and cheaper than lithium, and batteries powered by sodium-ion intercalation are increasingly seen as a potential alternative to lithium-ion batteries.
In September 2015 Goodenough’s lab identified a new cathode material for sodium-ion batteries made from eldfellite (NaFe(SO4)2), a nontoxic and inexpensive mineral, and Goodenough continues to experiment with different solid electrolytes so he can completely “eliminate the dendrite problem.”
“We’re on a cusp where a lot of things are starting to come together,” he says, optimistic that the next major advance in battery technology could come within the next year. “It’s an exciting time.”
